Brain and heart disorders explained by mutations in genes that regulate voltage-gated sodium channels
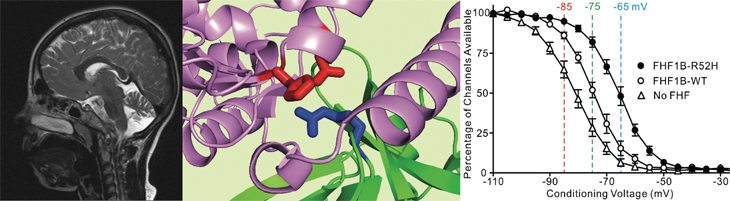
Electrical activity in brain and heart cells consists of action potentials driven by the opening and inactivation of voltage-gated sodium channels. Hunter College Professor Mitchell Goldfarb and graduate students Yue Liu, Christopher Marra and Katarzyna Dover in the CUNY Graduate Center Biology Program and Neuroscience Collaborative have been researching a set of sodium channel-binding proteins called FHFs that modulate sodium channel inactivation and consequent action potential generation and conduction. Three 2016 papers published in Nature Communications and Neurology by the Goldfarb lab in collaboration with those of Dejan Zecevic (Yale), Glenn Fishman (NYU), Egidio D’Angelo (U. Pavia), and Gunnar Buyse (U. Leuven) show how FHF activity is essential for normal brain and heart function and how altered FHF activity can induce neurological and cardiac disorders. In Siekierska et al, Neurology 86:2162, an FHF1 point mutation that subtly alters sodium channel binding and enhances inactivation modulation was identified in two siblings who suffered early onset epileptic encephalopathy. Additional clinical examples of the same genetic mutation have since been described elsewhere, thereby defining an FHF1 Epileptic Encephalopathy Syndrome. In Dover et al, Nature Communications 7:12895, FHFs in brain neurons are shown to be associated with sodium channels that initiate action potentials, but far less so with sodium channels in the same cells that mediate spike conduction along axons. These findings have implications for how thin axons with limited chemical energy reserves can sustain conduction of high frequency spiking. In Park et al, Nature Communications 7:12966, the gene encoding FHF2 is shown to be essential for action potential conduction through heart muscle. Mice with a disrupted Fhf2 gene experience cardiac conduction slowing and arrest under hyperthermic stress, providing critical new insight into the physiological mechanism of fever-associated cardiac conduction failure.
- Siekierska et al. Neurology 86:2162 (2016) http://www.neurology.org/content/86/23/2162.long
- Dover et al. Nature Communications 7:12895 (2016) http://www.nature.com/articles/ncomms12895
- Park et al. Nature Communications 7:12966 (2016) http://www.nature.com/articles/ncomms12966
